Galapagos are developing new CFTR potentiators and correctors. The image below suggests that there is superior CFTR opening with their potentiator (GLPG1) compared to Kalydeco with F508del, and that GLPG1 may be more potent with G551D than Kalydeco.
Galapagos has a “large pipeline of four clinical, seven pre-clinical, and 30 discovery small-molecule and antibody programs in cystic fibrosis, inflammation, antibiotics, metabolic disease, and other indications.”
“Successful collaboration with CF Foundation
– First disease in which Galapagos would discover, develop and launch its own medicines
– Bring best in class potentiator to G551D population
– Progress correctors in discovery to address the F508 population
– Galapagos in strong position with own potentiator”
Note: Potentiators will hopefully help CFTR at the surface to open (ie class 3, 4, 5 mutations) and hopefully also work in conjunction with correctors for F508del. They plan to reach the pre-clinical stage this year.
Source: http://www.glpg.com/index.php/newspress-releasespress-releases/press-releases/placement/#1366706212
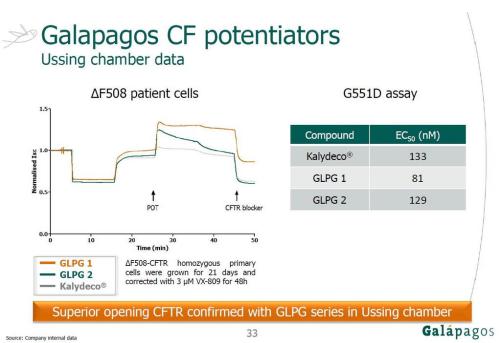
My interpretation of the image above: F508del:
1) the F508del cells have been treated with VX809 (hence some CFTR at the surface) before the start of the time in the graph,
2) the three potentiators are added at about 25min,
3) kalydeco shows the smallest improvement in terms of cftr opening, GLPG2 improves a lot but this is not sustained and basically gets back to the same point as kalydeco, and GLPG1 has a large improvement that is sustained,
4) after 45min a CFTR blocker is added so CFTR function decreases,
5) I am not sure exactly what happened at 5min and 15min, but I’m guessing it is part of setting up the experiment conditions (probably amiloride then forskolin).
G551D: I believe the lower EC50 number for GLPG1 indicates that it is more potent/has a better binding affinity with G551D than Kalydeco and GLPG2.
I have started a new blog about CF research, which has posts like this in it: http://sixtyfiverosesblog.wordpress.com/
Galapagos has a “large pipeline of four clinical, seven pre-clinical, and 30 discovery small-molecule and antibody programs in cystic fibrosis, inflammation, antibiotics, metabolic disease, and other indications.”
“Successful collaboration with CF Foundation
– First disease in which Galapagos would discover, develop and launch its own medicines
– Bring best in class potentiator to G551D population
– Progress correctors in discovery to address the F508 population
– Galapagos in strong position with own potentiator”
Note: Potentiators will hopefully help CFTR at the surface to open (ie class 3, 4, 5 mutations) and hopefully also work in conjunction with correctors for F508del. They plan to reach the pre-clinical stage this year.
Source: http://www.glpg.com/index.php/newspress-releasespress-releases/press-releases/placement/#1366706212

My interpretation of the image above: F508del:
1) the F508del cells have been treated with VX809 (hence some CFTR at the surface) before the start of the time in the graph,
2) the three potentiators are added at about 25min,
3) kalydeco shows the smallest improvement in terms of cftr opening, GLPG2 improves a lot but this is not sustained and basically gets back to the same point as kalydeco, and GLPG1 has a large improvement that is sustained,
4) after 45min a CFTR blocker is added so CFTR function decreases,
5) I am not sure exactly what happened at 5min and 15min, but I’m guessing it is part of setting up the experiment conditions (probably amiloride then forskolin).
G551D: I believe the lower EC50 number for GLPG1 indicates that it is more potent/has a better binding affinity with G551D than Kalydeco and GLPG2.
I have started a new blog about CF research, which has posts like this in it: http://sixtyfiverosesblog.wordpress.com/
